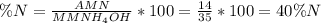Chemistry, 29.07.2019 14:00 antoinewill05
The percent composition by mass of nitrogen in nh4oh(gram formula mass= 35 grams/mole) is equal to which of the following? a.4/35×100. b.14/35×100 c.35/14×100 d.35/4×100

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
The percent composition by mass of nitrogen in nh4oh(gram formula mass= 35 grams/mole) is equal to w...
Questions

Mathematics, 04.08.2019 14:00


Mathematics, 04.08.2019 14:00


Social Studies, 04.08.2019 14:00

History, 04.08.2019 14:00

World Languages, 04.08.2019 14:00



Mathematics, 04.08.2019 14:00




History, 04.08.2019 14:00


History, 04.08.2019 14:00


History, 04.08.2019 14:00

English, 04.08.2019 14:00