Chemistry, 29.07.2019 17:30 jagmeetcheema
Write balanced complete ionic equation for the reaction agno3(aq)+kcl(aq)→agcl(s)+kno3(aq).

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
Write balanced complete ionic equation for the reaction agno3(aq)+kcl(aq)→agcl(s)+kno3(aq)....
Questions


Biology, 05.10.2019 08:00


History, 05.10.2019 08:00


Computers and Technology, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00

History, 05.10.2019 08:00

Chemistry, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00



English, 05.10.2019 08:00


English, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00

Physics, 05.10.2019 08:00


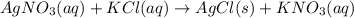
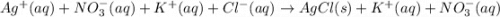 ....... (1)
....... (1)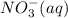 and
and 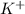 from both left and right side of the equation (1).
from both left and right side of the equation (1).