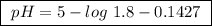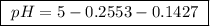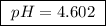Chemistry, 29.07.2019 21:30 blakestuhan
Calculate the ph of a buffer that is 0.225 m hc2h3o2 and 0.162 m kc2h3o2. the ka for hc2h3o2 is 1.8 ã 10-5. 4.60 9.26 4.74 4.89 9.11

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Calculate the ph of a buffer that is 0.225 m hc2h3o2 and 0.162 m kc2h3o2. the ka for hc2h3o2 is 1.8...
Questions

English, 29.08.2019 12:00


History, 29.08.2019 12:00

Social Studies, 29.08.2019 12:00

SAT, 29.08.2019 12:00

History, 29.08.2019 12:00

Social Studies, 29.08.2019 12:00


Geography, 29.08.2019 12:00



Mathematics, 29.08.2019 12:00





Business, 29.08.2019 12:00



Health, 29.08.2019 12:00

![[HC_2H_3O_2]=0.225 M](/tpl/images/0148/0955/5714c.png)
![[KC_2H_3O_2]=0.162 M](/tpl/images/0148/0955/ed9ec.png)
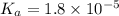
![pH=pK_a+\log\frac{[salt]}{[acid]}](/tpl/images/0148/0955/2a3b3.png)
![pH=-\log[1.8 \times 10^{-5}]+\log\frac{0.162 M}{0.225 M}](/tpl/images/0148/0955/31d23.png)
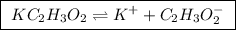
![\boxed{ \ pH = pK_a + log\frac{[A^-]}{[HA]} \ }](/tpl/images/0148/0955/a4470.png)
![\boxed{ \ pH = pK_a + log\frac{[C_2H_3O_2^-]}{[HC_2H_3O_2]} \ }](/tpl/images/0148/0955/cb27c.png)
![\boxed{ \ pH = -log(1.8 \times 10^{-5}) + log\frac{[0.162]}{[0.225]} \ }](/tpl/images/0148/0955/9cc5c.png)