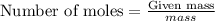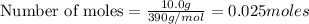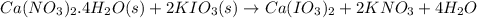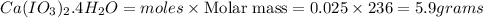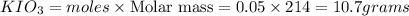Chemistry, 29.07.2019 22:00 tatilynnsoto17
Calculate the masses of ca(no3)2•4h2o(s) and kio3(s) required to make 10.0 g of ca(io3)2(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Calculate the masses of ca(no3)2•4h2o(s) and kio3(s) required to make 10.0 g of ca(io3)2(s)...
Questions

English, 10.11.2019 21:31





Mathematics, 10.11.2019 21:31


Biology, 10.11.2019 21:31

Mathematics, 10.11.2019 21:31






English, 10.11.2019 21:31

History, 10.11.2019 21:31



Mathematics, 10.11.2019 21:31

English, 10.11.2019 21:31

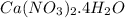 and
and 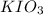 required to make 10.0 g of
required to make 10.0 g of 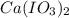 is 5.9 and 10.7 grams respectively.
is 5.9 and 10.7 grams respectively.