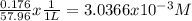Chemistry, 30.07.2019 01:30 Alexandragurule18
Calculate the external pressure that must be applied to seawater, 1.14 m total ion concentration at 10 degrees c if the maximum concentration allowed in the product water is 176 mg/l. assume that all the dissolved salts in the product water is sodium chloride. so, i know that i need to subtract the ion concentrations before using the pi=mrt formula, but i can't figure out how to convert mg/l into molarity. !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Calculate the external pressure that must be applied to seawater, 1.14 m total ion concentration at...
Questions

Geography, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00

History, 21.10.2019 18:00



Mathematics, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00


Biology, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00

Social Studies, 21.10.2019 18:00


Social Studies, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00



History, 21.10.2019 18:00

Mathematics, 21.10.2019 18:00