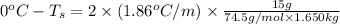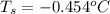Chemistry, 30.07.2019 02:00 melinda12ms
Calculate the freezing point of a solution containing 15 grams of kcl and 1650.0 grams of water. the molal-freezing-point-depression constant (kf) for water is 1.86 ∘c/m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

You know the right answer?
Calculate the freezing point of a solution containing 15 grams of kcl and 1650.0 grams of water. the...
Questions








Mathematics, 25.06.2019 09:10




Health, 25.06.2019 09:10


Spanish, 25.06.2019 09:10






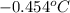
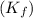 for water =
for water = 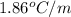
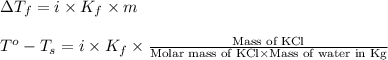
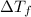 = change in freezing point
= change in freezing point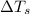 = freezing point of solution = ?
= freezing point of solution = ?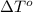 = freezing point of water =
= freezing point of water = 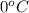
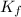 = freezing point constant for water =
= freezing point constant for water =