
Chemistry, 30.07.2019 15:30 jacobdobson5856
Why does water (h2o) have a significantly higher boiling point than methane (ch4)? hydrogen forms a stronger covalent bond with oxygen than with carbon. hydrogen forms a weaker covalent bond with oxygen than with carbon. water molecules are attracted to one another by hydrogen bonds. water molecules are made of fewer atoms than methane molecules.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Why does water (h2o) have a significantly higher boiling point than methane (ch4)? hydrogen forms a...
Questions

English, 18.10.2020 21:01


Mathematics, 18.10.2020 21:01



History, 18.10.2020 21:01


Geography, 18.10.2020 21:01


Chemistry, 18.10.2020 21:01

Spanish, 18.10.2020 21:01



Mathematics, 18.10.2020 21:01




History, 18.10.2020 21:01


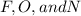 ) and the lone pair electrons present in the other molecule on these electronegative atoms is known as hydrogen bonding.
) and the lone pair electrons present in the other molecule on these electronegative atoms is known as hydrogen bonding.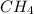 is Van der Waals interactions whereas in water,
is Van der Waals interactions whereas in water, 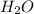 is hydrogen bonding.
is hydrogen bonding.

