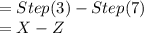Terry, a student, performs a titration. he completes these steps as part of his titration procedure: 1. he cleans and rinses a burette with standardized base. 2. he fills the burette with standardized base solution. 3. he reads and records the initial burette volume. 4. he adds a base from the burette to an acid. 5. he observes a color change in the erlenmeyer flask. 6. he stops the addition of base from the burette. 7. he reads and records the final burette volume. which steps will provide information needed to calculate the volume of base needed to reach the equivalence point? 1 and 6, 3 and 7, 3, 4, and 6, or 1, 2, and 7?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

You know the right answer?
Terry, a student, performs a titration. he completes these steps as part of his titration procedure:...
Questions




Biology, 04.07.2019 04:30


History, 04.07.2019 04:30

Biology, 04.07.2019 04:30

Mathematics, 04.07.2019 04:30

History, 04.07.2019 04:30





Social Studies, 04.07.2019 04:30

Computers and Technology, 04.07.2019 04:30


Mathematics, 04.07.2019 04:30


Mathematics, 04.07.2019 04:30

![= X\\[/tex ----------------Step (3)Now the final volume in burette after adding the base to the acid solution to attain equivalence point [tex]= Z\\](/tpl/images/0151/0895/41313.png) ----------------Step (7)
----------------Step (7)