
Chemistry, 31.07.2019 06:30 smusisca53
Calculate the vapor pressure of a solution of 0.99 mol of cholesterol in 5.4 mol of toluene at 32°c. pure toluene has a vapor pressure of 41 torr at 32°c. (assume ideal behavior.)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Calculate the vapor pressure of a solution of 0.99 mol of cholesterol in 5.4 mol of toluene at 32°c....
Questions


Mathematics, 29.10.2020 21:00


English, 29.10.2020 21:00




Biology, 29.10.2020 21:00

History, 29.10.2020 21:00


Mathematics, 29.10.2020 21:00


History, 29.10.2020 21:00

English, 29.10.2020 21:00

Mathematics, 29.10.2020 21:00

French, 29.10.2020 21:00

Mathematics, 29.10.2020 21:00



Mathematics, 29.10.2020 21:00

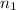 = 0.99 mol
= 0.99 mol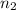 = 5.4 mol
= 5.4 mol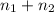
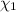 ) is as follows.
) is as follows.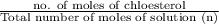
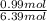
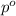 ) = 41 torr
) = 41 torr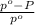
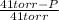 = 0.154
= 0.154 

