
Chemistry, 31.07.2019 15:30 jhunruh6247
The ka of benzoic acid is 6.4 x 10–5. what is the approximate ph of a 1.5 m solution of benzoic acid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1
You know the right answer?
The ka of benzoic acid is 6.4 x 10–5. what is the approximate ph of a 1.5 m solution of benzoic acid...
Questions



Mathematics, 07.11.2020 01:00






Spanish, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00



History, 07.11.2020 01:00



Mathematics, 07.11.2020 01:00



Mathematics, 07.11.2020 01:00

Chemistry, 07.11.2020 01:00

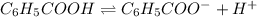
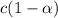
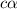
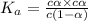
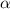 is very very small the, the expression will be,
is very very small the, the expression will be,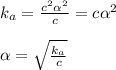
![[H^+]=c\alpha](/tpl/images/0154/8519/21a04.png)
![[H^+]=\sqrt{k_a\times c}](/tpl/images/0154/8519/881de.png)
![[H^+]=\sqrt{(6.4\times 10^{-5})\times 1.5}](/tpl/images/0154/8519/407b7.png)
![[H^+]=9.7\times 10^{-3}M](/tpl/images/0154/8519/1d5ea.png)
![pH=-log[H^+]](/tpl/images/0154/8519/15713.png)
![pH=-log[9.7\times 10^{-3}]=2.01](/tpl/images/0154/8519/353eb.png)


