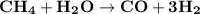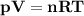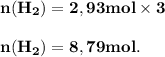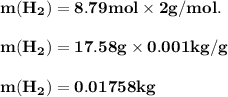Chemistry, 31.07.2019 18:30 katlynnschmolke
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. suppose a chemical engineer studying a new catalyst for the reform reaction finds that 159. liters per second of methane are consumed when the reaction is run at 294.°c and 0.86atm . calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3


Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of...
Questions


Mathematics, 21.08.2019 01:00


Mathematics, 21.08.2019 01:00

Mathematics, 21.08.2019 01:00

Mathematics, 21.08.2019 01:00

History, 21.08.2019 01:00


History, 21.08.2019 01:00

Physics, 21.08.2019 01:00

Social Studies, 21.08.2019 01:00




English, 21.08.2019 01:00

English, 21.08.2019 01:00

Mathematics, 21.08.2019 01:00



Biology, 21.08.2019 01:00