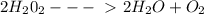Chemistry, 01.08.2019 00:30 justritegrl
In the reaction below, hydrogen peroxide decomposes to water. mm h2o2 = 34.02 g/mol mm h2o = 18.02 g/mol mm o2 = 32 g/mol 2h2o2 → 2h2o + o2 if 14.3 moles of h2o2 is decomposed, how many grams of oxygen gas are produced? show all your work to get full credit.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

You know the right answer?
In the reaction below, hydrogen peroxide decomposes to water. mm h2o2 = 34.02 g/mol mm h2o = 18.02 g...
Questions

Mathematics, 20.09.2020 02:01


Geography, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01


History, 20.09.2020 02:01

Physics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01



English, 20.09.2020 02:01



Mathematics, 20.09.2020 02:01


Computers and Technology, 20.09.2020 02:01