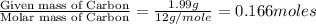Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 5.00 g of thi...
Questions



Medicine, 03.09.2020 23:01

English, 03.09.2020 23:01


History, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01




History, 03.09.2020 23:01



History, 03.09.2020 23:01

Physics, 03.09.2020 23:01


History, 03.09.2020 23:01

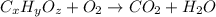
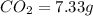
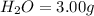
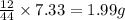 of carbon will be contained.
of carbon will be contained.