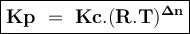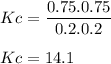Chemistry, 01.08.2019 16:30 afloareiandrei8615
An equilibrium mixture contains 0.750 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00 l container. co(g)+h2o(g)↽−−⇀co2(g)+h2(g) how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol once equilibrium has been reestablished?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
An equilibrium mixture contains 0.750 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions




Mathematics, 20.01.2021 02:00

English, 20.01.2021 02:00

English, 20.01.2021 02:00

Mathematics, 20.01.2021 02:00


History, 20.01.2021 02:00

English, 20.01.2021 02:00

Mathematics, 20.01.2021 02:00

Spanish, 20.01.2021 02:00

English, 20.01.2021 02:00



Computers and Technology, 20.01.2021 02:00



Mathematics, 20.01.2021 02:00

![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0158/5375/9d1db.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/0158/5375/b3cf6.png)