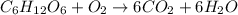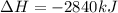Chemistry, 01.08.2019 16:30 carlalopezelox2244
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), according to the equation below. mc020-1.jpg the enthalpy of the reaction is –2,840 kj. what is the heat of combustion, per mole, of glucose? –2,840 kj/mol –473.3 kj/mol 473.3 kj/mol 2,840 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

You know the right answer?
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), acco...
Questions

Mathematics, 12.04.2021 09:50





Geography, 12.04.2021 09:50


Mathematics, 12.04.2021 09:50

Mathematics, 12.04.2021 09:50

Biology, 12.04.2021 09:50


Mathematics, 12.04.2021 09:50




History, 12.04.2021 09:50

English, 12.04.2021 09:50

Advanced Placement (AP), 12.04.2021 09:50


Social Studies, 12.04.2021 09:50