
Chemistry, 02.08.2019 09:30 aliyahlknox881
Afirst-order reaction has a half-life of 22.9 s . how long does it take for the concentration of the reactant in the reaction to fall to one-sixteenth of its initial value

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
Afirst-order reaction has a half-life of 22.9 s . how long does it take for the concentration of the...
Questions




English, 19.09.2020 01:01


Physics, 19.09.2020 01:01

Health, 19.09.2020 01:01






English, 19.09.2020 01:01



Chemistry, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01


Mathematics, 19.09.2020 01:01

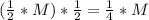
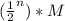
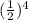 , we have that 4 half-lifes need to pass so that the concentration of the reactant becomes 1/16 of the initial one (=1/16*M). Hence, 4*22.9 sec=91.6 seconds is the required time.
, we have that 4 half-lifes need to pass so that the concentration of the reactant becomes 1/16 of the initial one (=1/16*M). Hence, 4*22.9 sec=91.6 seconds is the required time.

