

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Compared to the freezing point of 1.0 m kcl(aq) at standard pressure, the freezing point of 1.0 m ca...
Questions

Mathematics, 21.01.2021 20:20

Mathematics, 21.01.2021 20:20





Biology, 21.01.2021 20:20




English, 21.01.2021 20:20



Mathematics, 21.01.2021 20:20


Mathematics, 21.01.2021 20:20




English, 21.01.2021 20:20

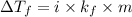
 = change in freezing point
= change in freezing point
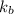 = freezing point constant
= freezing point constant
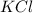 will be,
will be,
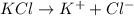
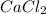 will be,
will be,
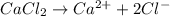
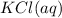 at standard pressure, the freezing point of 1.0 M
at standard pressure, the freezing point of 1.0 M 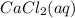 at standard pressure is lower.
at standard pressure is lower.

