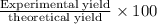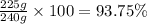In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulfur trioxide. using the equation, 2so2 (g) + o2 imported asset 2so3 (g), if 192 g of sulfur dioxide is given the opportunity to react with an excess of oxygen to produce 225 g of sulfur trioxide, what is the percent yield of this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulf...
Questions


Biology, 30.10.2021 01:00


Biology, 30.10.2021 01:00



Mathematics, 30.10.2021 01:00


Mathematics, 30.10.2021 01:00


Mathematics, 30.10.2021 01:00

Mathematics, 30.10.2021 01:00


Mathematics, 30.10.2021 01:00

Business, 30.10.2021 01:00

Social Studies, 30.10.2021 01:00


Social Studies, 30.10.2021 01:00


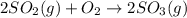
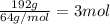
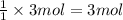 of sulfur trioxide
of sulfur trioxide