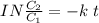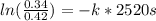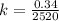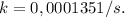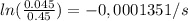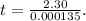Chemistry, 02.08.2019 15:30 unknown54321
It takes 42.0 min for the concentration of a reactant in a first–order reaction to drop from 0.45 m to 0.32 m at 25°c. how long will it take for the reaction to be 90% complete?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
It takes 42.0 min for the concentration of a reactant in a first–order reaction to drop from 0.45 m...
Questions

History, 25.07.2019 08:30

Physics, 25.07.2019 08:30

Mathematics, 25.07.2019 08:30




Mathematics, 25.07.2019 08:30


Mathematics, 25.07.2019 08:30


Mathematics, 25.07.2019 08:30