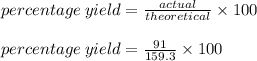Chemistry, 02.08.2019 16:00 slhfbfjcys
What is the percent yield of a reaction in which 200. g of phosphorus trichloride reacts with excess water to form 91.0 g of hcl and aqueous phosphorous acid (h3po3)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

You know the right answer?
What is the percent yield of a reaction in which 200. g of phosphorus trichloride reacts with excess...
Questions


Biology, 13.02.2020 22:04


Mathematics, 13.02.2020 22:04

Mathematics, 13.02.2020 22:05


World Languages, 13.02.2020 22:05



Mathematics, 13.02.2020 22:05





Mathematics, 13.02.2020 22:06




Biology, 13.02.2020 22:10