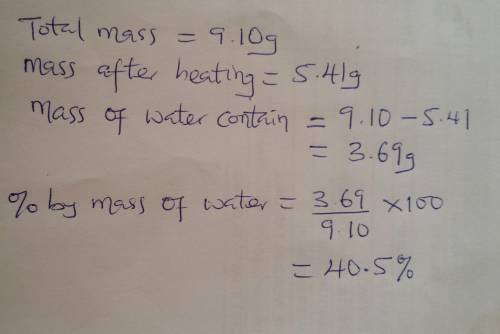Ahydrated salt is a solid that includes water molecules within its crystal structure. a student heated a 9.10-gram sample of a hydrated salt to
a constant mass of 5.41 grams. what percent by mass of water did the salt contain?
(1) 3.69% (3) 40.5%
(2) 16.8% (4) 59.5%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Ahydrated salt is a solid that includes water molecules within its crystal structure. a student heat...
Questions


Mathematics, 13.04.2021 17:20

Computers and Technology, 13.04.2021 17:20



Social Studies, 13.04.2021 17:20

Chemistry, 13.04.2021 17:20


Mathematics, 13.04.2021 17:20

Physics, 13.04.2021 17:20

Social Studies, 13.04.2021 17:20


Mathematics, 13.04.2021 17:20


Spanish, 13.04.2021 17:20


Mathematics, 13.04.2021 17:20

Arts, 13.04.2021 17:20