
Chemistry, 02.08.2019 22:30 rubimachuca1020
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.35 m, and the concentration of h2so3 is 0.23 m. this reaction a. is in equilibrium b. must shift to the reactants to be in equilibrium c. must shift to the products to be in equilibrium d. must have the pressure increased to reach equilibrium e. none of the above

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.3...
Questions






Geography, 11.10.2020 15:01

History, 11.10.2020 15:01


Advanced Placement (AP), 11.10.2020 15:01


English, 11.10.2020 15:01





Chemistry, 11.10.2020 15:01


History, 11.10.2020 15:01


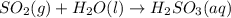
![Q=\frac{[H_2SO_3(aq)]}{[SO_2(g)]}](/tpl/images/0163/3606/a76c3.png)
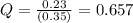
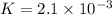
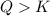 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.
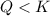 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.
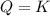 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

