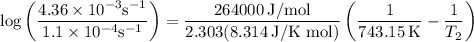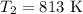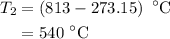Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
The rate constant of a reaction is 1.1 × 10-4 s-1 at 470 °c, and the activation energy is 264 kj/mol...
Questions



Mathematics, 03.11.2020 21:30

Health, 03.11.2020 21:30



Biology, 03.11.2020 21:30


Mathematics, 03.11.2020 21:30

Mathematics, 03.11.2020 21:30

Mathematics, 03.11.2020 21:30

English, 03.11.2020 21:30


Mathematics, 03.11.2020 21:30

English, 03.11.2020 21:30


Social Studies, 03.11.2020 21:30

Mathematics, 03.11.2020 21:30


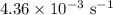 comes out to be
comes out to be  .
.
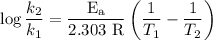 …… (1)
…… (1)
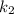 is rate constant at temperature
is rate constant at temperature 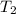 .
.
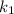 is rate constant temperature
is rate constant temperature 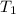 .
.
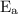 is activation energy.
is activation energy.
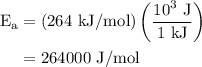
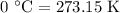
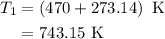
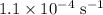 .
.