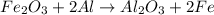Chemistry, 12.10.2019 01:40 minersaysay22
Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
during this reaction, the oxidation number of fe changes from
(1) +2 to 0 as electrons are transferred
(2) +2 to 0 as protons are transferred
(3) +3 to 0 as electrons are transferred
(4) +3 to 0 as protons are transferred

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2
You know the right answer?
Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
dur...
fe2o3 + 2al--> al2o3 + 2fe
dur...
Questions

Mathematics, 27.05.2020 22:06


English, 27.05.2020 22:06

English, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06



History, 27.05.2020 22:06

Mathematics, 27.05.2020 22:06

History, 27.05.2020 22:06

Geography, 27.05.2020 22:06