
Chemistry, 03.08.2019 03:00 anthonymcnulty6471
The water-gas shift reaction co(g)+h2o(g)⇌co2(g)+h2(g) is used industrially to produce hydrogen. the reaction enthalpy is δh∘=−41kj. to increase the equilibrium yield of hydrogen would you use high or low temperature?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
The water-gas shift reaction co(g)+h2o(g)⇌co2(g)+h2(g) is used industrially to produce hydrogen. the...
Questions

Health, 16.11.2020 14:20

Social Studies, 16.11.2020 14:20



Mathematics, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20


Business, 16.11.2020 14:20


Social Studies, 16.11.2020 14:20



Arts, 16.11.2020 14:20

French, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20



Mathematics, 16.11.2020 14:20

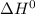 ) for water-gas shift reaction is negative.That means the given reaction is exothermic. So heat is produced in formation of
) for water-gas shift reaction is negative.That means the given reaction is exothermic. So heat is produced in formation of 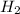 Hence decrease in temperature would lead to shift of equilibrium towards forward direction i.e. formation of
Hence decrease in temperature would lead to shift of equilibrium towards forward direction i.e. formation of  and
and 

