Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
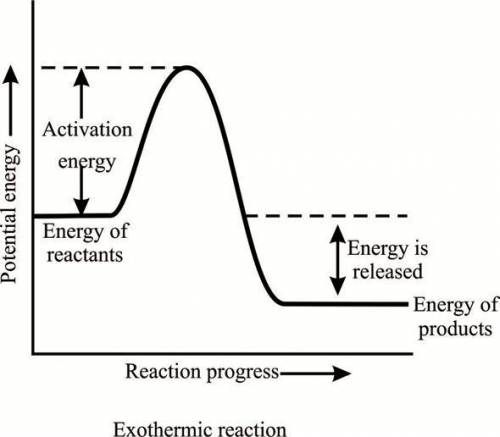
What is the meaning of a value of –400 kj in a reaction? the reaction requires 400 kj of heat. the...
Questions

Mathematics, 20.03.2020 00:01


Biology, 20.03.2020 00:01

Mathematics, 20.03.2020 00:01

Mathematics, 20.03.2020 00:01

Mathematics, 20.03.2020 00:01



Mathematics, 20.03.2020 00:01


Mathematics, 20.03.2020 00:01

Mathematics, 20.03.2020 00:01





Business, 20.03.2020 00:01