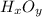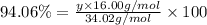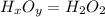Chemistry, 03.08.2019 12:00 rainbowboi
Acompound composed of only hydrogen and oxygen is 5.94% hydrogen by mass. the molar mass of this compound is 34.02 g/mol. what is the compound's molecular formula?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
You know the right answer?
Acompound composed of only hydrogen and oxygen is 5.94% hydrogen by mass. the molar mass of this com...
Questions


Mathematics, 25.05.2021 01:00




Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00




Chemistry, 25.05.2021 01:00

Social Studies, 25.05.2021 01:00

History, 25.05.2021 01:00


Mathematics, 25.05.2021 01:00

Social Studies, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Social Studies, 25.05.2021 01:00

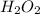 is the compound's molecular formula.
is the compound's molecular formula.