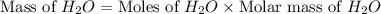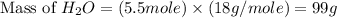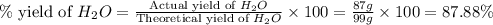Chemistry, 03.08.2019 17:00 akitchen10
Consider the chemical equation. 2h2 + o2 mc022-1.jpg 2h2o what is the percent yield of h2o if 87.0 g of h2o is produced by combining 95.0 g of o2 and 11.0 g of h2? use mc022-2.jpg.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Consider the chemical equation. 2h2 + o2 mc022-1.jpg 2h2o what is the percent yield of h2o if 87.0 g...
Questions

Biology, 10.07.2019 13:30

Biology, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30

Business, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30


History, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30

Computers and Technology, 10.07.2019 13:30





Mathematics, 10.07.2019 13:30


Mathematics, 10.07.2019 13:30



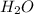 is, 87.88%
is, 87.88%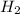 and
and 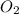 .
.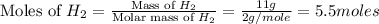
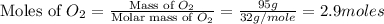
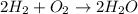
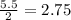 moles of
moles of 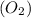 = 2.9 - 2.75 = 0.15 moles
= 2.9 - 2.75 = 0.15 moles