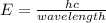Chemistry, 03.08.2019 17:30 rachelreed
When an electron of an atom falls from a higher energy level to the ground state, the atom loses 9.4145 x 10-25 joules of energy. what is the wavelength of the radiation emitted as a result of this transition? (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108m/s)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
When an electron of an atom falls from a higher energy level to the ground state, the atom loses 9.4...
Questions

English, 11.04.2021 22:40

Physics, 11.04.2021 22:40






Mathematics, 11.04.2021 22:50






Mathematics, 11.04.2021 22:50


History, 11.04.2021 22:50




Social Studies, 11.04.2021 22:50