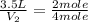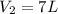The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(g). if a balloon was filled with 3.5l of ammonia gas and all of the gas decomposed, what would be the total volume of gas in the container? assume the pressure and temp remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 05:30
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b.cns c. ans d. pns
Answers: 1
You know the right answer?
The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(...
Questions

Chemistry, 01.09.2021 01:00




Mathematics, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00




Mathematics, 01.09.2021 01:00





Mathematics, 01.09.2021 01:00

Social Studies, 01.09.2021 01:00

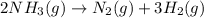
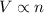
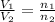
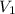 = initial volume of ammonia gas = 3.5 L
= initial volume of ammonia gas = 3.5 L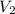 = total volume of gas in the container = ?
= total volume of gas in the container = ?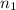 = initial moles of ammonia gas = 2 mole
= initial moles of ammonia gas = 2 mole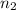 = final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles
= final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles