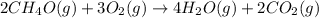Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The combustion of methanol (ch4o) is described by this balanced chemical equation: 2ch4o(g) + 3o2(g...
Questions

Mathematics, 29.04.2021 14:00


History, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00

English, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00




Mathematics, 29.04.2021 14:00


Mathematics, 29.04.2021 14:00

Spanish, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00

Social Studies, 29.04.2021 14:00


Business, 29.04.2021 14:00

Computers and Technology, 29.04.2021 14:00