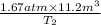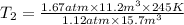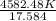Chemistry, 26.09.2019 07:30 mediocresquash
The volume of a gas decreases from 15.7 m3 to 11.2 m3 while the pressure changes from 1.12 atm to 1.67 atm. if the initial temperature is 245 k, what is the final temperature of the gas

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
The volume of a gas decreases from 15.7 m3 to 11.2 m3 while the pressure changes from 1.12 atm to 1....
Questions



Mathematics, 30.01.2020 13:57

Mathematics, 30.01.2020 13:57

History, 30.01.2020 13:57


English, 30.01.2020 13:57



Mathematics, 30.01.2020 13:57

Mathematics, 30.01.2020 13:57

Chemistry, 30.01.2020 13:57

English, 30.01.2020 13:57

Mathematics, 30.01.2020 13:57

Mathematics, 30.01.2020 13:57

Biology, 30.01.2020 13:57

Mathematics, 30.01.2020 13:57



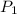 = 1.12 atm,
= 1.12 atm, 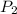 = 1.67 atm
= 1.67 atm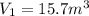 ,
, 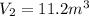
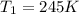 ,
, 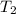 = ?
= ?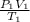 =
= 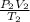
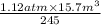 =
=