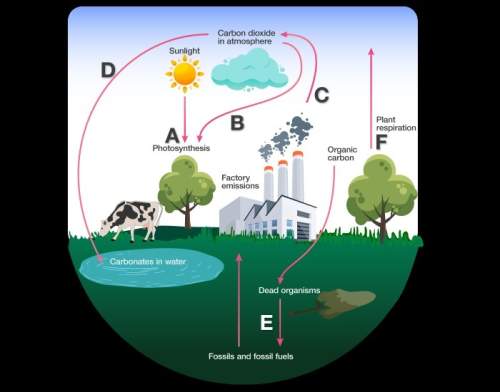
Using the diagram above, answer the following questions:
6. true or false. the arrow labeled...

Chemistry, 13.10.2019 00:30 lopezsharon333
Using the diagram above, answer the following questions:
6. true or false. the arrow labeled c represents a transfer of chemical energy to mechanical energy. explain why this is true or false.
7. true or false. the arrow labeled a represents a transfer of solar energy to chemical energy. explain why this is true or false.
8. which arrow or arrows represent a release of carbon dioxide? what process is occurring at the arrow(s) you selected?
9. which arrow or arrows indicate a process that cycles carbon from living or nonliving organisms? describe the process or processes you selected.
10. which arrow or arrows represent reactions that demonstrate a conservation of mass and energy? explain your answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Questions


Chemistry, 07.07.2021 14:00



Chemistry, 07.07.2021 14:00


Biology, 07.07.2021 14:00



Physics, 07.07.2021 14:00

Mathematics, 07.07.2021 14:00

Biology, 07.07.2021 14:00



English, 07.07.2021 14:00


English, 07.07.2021 14:00

Computers and Technology, 07.07.2021 14:00


Mathematics, 07.07.2021 14:00



