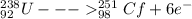proton produces nuclide x and a neutron. what is nuclide x?

Chemistry, 25.08.2019 01:30 lathwkuster
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
answers:
magnesium-24
magnesium-23
neon-23
s
odium-24
none of the above
the isotope p
has a half-life of 14.3 days. if a sample originally contained 1.00 g of p,
how much was left after 43 days?
answers:
0.250 g
0.125 g
0.750 g
0.500 g
identify x in the reaction
below.
u
+ c
→ cf
+ x
answers:
1
alpha particle
3
protons
6
neutrons
6
electrons
a .20 gram sample of c-14 was allowed to decay for 3 half-lives. what mass
of the sample will remain? carbon-14 has a half life of 5730
years.
answers:
0.025
0.05
0.1
0
.01
0.05
the isotope cu
has a half-life of 30 s. if a sample originally contained 48 mg of cu,
how much time passed before the amount fell to 3 mg?
answers:
120 s
240s
30 s
60 s(when i did my calculation for the question above, i got 60 seconds)
what radionuclide decays to fe-56
by beta emission?
answers:
fe
co
mn
co
mnhow do i know wat it becomes. i put 57 co 27 and it's wrong.
the cf
to cf
conversion is accompanied by
answers:
an alpha
emission
a
neutron capture
an electron
capture
an electron releasei would greatly appreciate your . you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
proton produces nuclide x and a neutron. what is nuclide x?
Questions






Mathematics, 12.08.2020 04:01

English, 12.08.2020 04:01



Mathematics, 12.08.2020 04:01





English, 12.08.2020 04:01





Social Studies, 12.08.2020 04:01