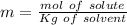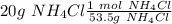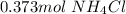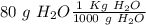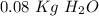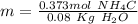Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Calculate the molality of a 20.0 percent by weight aqueous solution of nh4cl. (molecular weight: nh...
Questions



English, 24.12.2020 08:20

Mathematics, 24.12.2020 08:20

World Languages, 24.12.2020 08:20

Mathematics, 24.12.2020 08:20


Computers and Technology, 24.12.2020 08:30

Social Studies, 24.12.2020 08:30



Mathematics, 24.12.2020 08:30


English, 24.12.2020 08:30


Mathematics, 24.12.2020 08:30


Mathematics, 24.12.2020 08:30


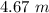
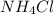 . So, in the 100 g of solution we will have 80 g of
. So, in the 100 g of solution we will have 80 g of 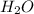 (100-20= 80). If we remember the molality equation:
(100-20= 80). If we remember the molality equation: