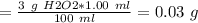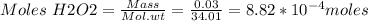Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
Assuming the density of 3% h2o2 is 1.00g/ml, what mass of h2o2 is in a 1.00ml sample? how many mole...
Questions

Mathematics, 25.02.2020 02:50


History, 25.02.2020 02:51


Computers and Technology, 25.02.2020 02:51






English, 25.02.2020 02:51

Computers and Technology, 25.02.2020 02:51


Mathematics, 25.02.2020 02:51

Spanish, 25.02.2020 02:51

Health, 25.02.2020 02:51


Computers and Technology, 25.02.2020 02:51