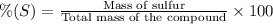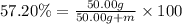Chemistry, 05.08.2019 01:00 heavendl13
Oxygen forms a number of compounds with sulfur, many of which are quite reactive. one such compound is 57.20 % by mass s . calculate the number of grams of oxygen present in a sample of this compound that contains 50.00 grams of s .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
Oxygen forms a number of compounds with sulfur, many of which are quite reactive. one such compound...
Questions


History, 31.03.2020 03:24

Geography, 31.03.2020 03:24

History, 31.03.2020 03:24

Mathematics, 31.03.2020 03:24






History, 31.03.2020 03:24



Mathematics, 31.03.2020 03:25




Mathematics, 31.03.2020 03:25