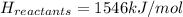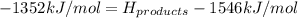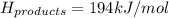Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants is 1546 kj/mol. calculate the total bonding energy of the products and decide whether the reaction is endothermic or exothermic a)84 kj/mol, endothermic b)194 kj/mol, endothermic c) 84 kj/mol, exothermic d)194 kj/mol, exothermic

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3


Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants...
Questions


Mathematics, 25.02.2021 14:00

English, 25.02.2021 14:00


Mathematics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00


Computers and Technology, 25.02.2021 14:00

English, 25.02.2021 14:00

English, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

Biology, 25.02.2021 14:00

History, 25.02.2021 14:00



Mathematics, 25.02.2021 14:00


Biology, 25.02.2021 14:00

Medicine, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

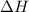 for the reaction comes out to be negative.
for the reaction comes out to be negative.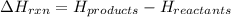
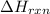 = 1352kJ/mol[/tex]
= 1352kJ/mol[/tex]