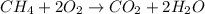Chemistry, 04.08.2019 21:40 Chandler1Gaming
Methane combines with oxygen in the air to make carbon dioxide and water vapor. which of the chemical equations matches the reaction above? a. ch4 + 2o2 co2 + 2h2o b. c2h4 + 2o2 2co + 2h2o c. ch4 + 2co2 2co2 + 2h2o d. ch4 + 2o 2co + 2h2o

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

You know the right answer?
Methane combines with oxygen in the air to make carbon dioxide and water vapor. which of the chemica...
Questions






Advanced Placement (AP), 17.07.2020 20:01

English, 17.07.2020 20:01


Mathematics, 17.07.2020 20:01

Biology, 17.07.2020 20:01








Biology, 17.07.2020 20:01

Mathematics, 17.07.2020 20:01

Computers and Technology, 17.07.2020 20:01