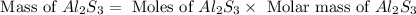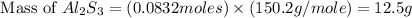Chemistry, 20.08.2019 02:30 leeenaaa95
How many grams of aluminum sulfide can form from the reaction 9.00 g of aluminum with 8.00 g of sulfur

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
How many grams of aluminum sulfide can form from the reaction 9.00 g of aluminum with 8.00 g of sulf...
Questions

Biology, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30


Mathematics, 17.05.2021 22:30

English, 17.05.2021 22:30

English, 17.05.2021 22:30


English, 17.05.2021 22:30

Social Studies, 17.05.2021 22:30


Computers and Technology, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30

Mathematics, 17.05.2021 22:30


Mathematics, 17.05.2021 22:30


Health, 17.05.2021 22:30

Chemistry, 17.05.2021 22:30

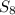 = 8.00 g
= 8.00 g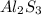 = 150.2 g/mole
= 150.2 g/mole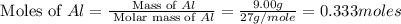
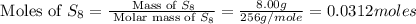
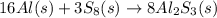
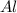
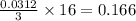 moles of
moles of 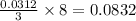 moles of
moles of