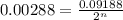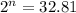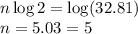Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Ameteorite contains 0.17 g of nickel-59, a radioisotope that decays to form cobalt-59. the meteorite...
Questions


Mathematics, 12.10.2020 21:01

Business, 12.10.2020 21:01

Advanced Placement (AP), 12.10.2020 21:01

Mathematics, 12.10.2020 21:01




Mathematics, 12.10.2020 21:01

English, 12.10.2020 21:01





English, 12.10.2020 21:01

Chemistry, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01


History, 12.10.2020 21:01

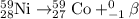
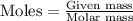
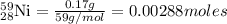
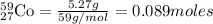
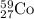 is produced by 1 mole
is produced by 1 mole 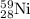
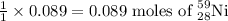
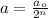
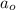 = Initial amount of the reactant = 0.09188 moles
= Initial amount of the reactant = 0.09188 moles