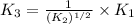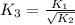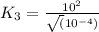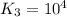Chemistry, 02.08.2019 06:40 kenldykido2300
For the hypothetical reactions 1 and 2, k1 = 102 and k2 = 10–4. 1. a2(g) + b2(g) 2ab(g) 2. 2a2(g) + c2(g) 2a2c(g) 3. a2c(g) + b2(g) 2ab(g) + (1/2)c2(g) what is the value for k for reaction 3?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
For the hypothetical reactions 1 and 2, k1 = 102 and k2 = 10–4. 1. a2(g) + b2(g) 2ab(g) 2. 2a2(g) +...
Questions

Mathematics, 27.08.2019 13:00

History, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00


Mathematics, 27.08.2019 13:00



Mathematics, 27.08.2019 13:00

English, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00



English, 27.08.2019 13:00


Mathematics, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00

Mathematics, 27.08.2019 13:00



History, 27.08.2019 13:00

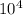
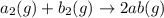 ;
; 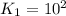
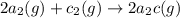 ;
; 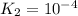
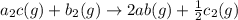
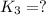
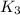 for the final reaction.
for the final reaction.