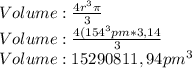Chemistry, 01.08.2019 05:00 Tyrant4life
Given the atomic radius of neon, 0.69 å, and knowing that a sphere has a volume of 4πr3/3, calculate the fraction of space that ne atoms occupy in a sample of neon at stp.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Given the atomic radius of neon, 0.69 å, and knowing that a sphere has a volume of 4πr3/3, calculate...
Questions


Mathematics, 04.03.2021 18:20



Mathematics, 04.03.2021 18:20

French, 04.03.2021 18:20

Advanced Placement (AP), 04.03.2021 18:20





Mathematics, 04.03.2021 18:20

English, 04.03.2021 18:20

Mathematics, 04.03.2021 18:20





Arts, 04.03.2021 18:20