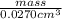Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
The density of carbon in the form of diamond is 3.51 g/cm^3. if you have a small diamond with a volu...
Questions

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00



Biology, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00

English, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Chemistry, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00



Mathematics, 13.05.2021 01:00

Advanced Placement (AP), 13.05.2021 01:00

Mathematics, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00

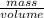
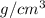 and volume is 0.0270
and volume is 0.0270 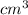 .
.