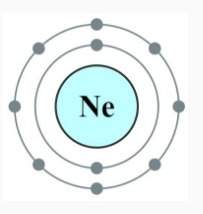
This is a model of a neon atom.
how likely is it that this atom would want to bond with...

Chemistry, 30.12.2019 20:31 Manuel2019
This is a model of a neon atom.
how likely is it that this atom would want to bond with another atom?
a. this atom is very likely to bond with another atom.
b. this atom is only slightly likely to bond with another atom
c. this atom is not likely at all to bond with another atom.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Questions




Mathematics, 03.06.2021 06:20

Biology, 03.06.2021 06:20




Mathematics, 03.06.2021 06:20


History, 03.06.2021 06:30

Mathematics, 03.06.2021 06:30


English, 03.06.2021 06:30

Mathematics, 03.06.2021 06:30


Social Studies, 03.06.2021 06:30





