
Chemistry, 29.07.2019 19:00 elitehairnerd1964
Which of the following best explains the polarity of water (h2o)? a. protons are more attracted to the hydrogen atoms, so the hydrogen atoms become positively charged. b. since the oxygen atom has more electrons than the hydrogen atoms, it is negatively charged. c. electrons are more attracted to the oxygen atom, so there is a partial negative charge on the oxygen atom. d. since there are two positive hydrogen atoms and only one negative oxygen atom, the water molecule is positively charged.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
Which of the following best explains the polarity of water (h2o)? a. protons are more a...
Questions


Mathematics, 30.03.2020 04:00


Mathematics, 30.03.2020 04:01

Chemistry, 30.03.2020 04:03

Mathematics, 30.03.2020 04:09





History, 30.03.2020 04:09

Mathematics, 30.03.2020 04:09


Mathematics, 30.03.2020 04:09


Mathematics, 30.03.2020 04:09

Mathematics, 30.03.2020 04:09

Mathematics, 30.03.2020 04:09


Mathematics, 30.03.2020 04:10

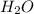 . This shows that there are two hydrogen atoms and one oxygen atom.
. This shows that there are two hydrogen atoms and one oxygen atom. 

