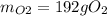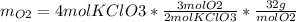The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info: mm o2 = 32 g/mol mm kcl = 74.55 g/mol mm kclo3 = 122.55 g/mol if 4.00 moles of kclo3 are totally consumed, how many grams of oxygen gas would be produced? 192 g 6.00 g 85.3 g 735 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info:...
Questions

Mathematics, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00




Biology, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00

Mathematics, 08.04.2021 23:00


Geography, 08.04.2021 23:00


English, 08.04.2021 23:00


Mathematics, 08.04.2021 23:00