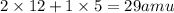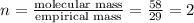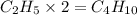Chemistry, 28.07.2019 13:50 natjojo0512
The empirical formula for a compound is c2h5 and its formula mass is 58 amu. what is its molecular formula?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
The empirical formula for a compound is c2h5 and its formula mass is 58 amu. what is its molecular f...
Questions


English, 26.06.2019 01:20


English, 26.06.2019 01:20

Mathematics, 26.06.2019 01:20




Biology, 26.06.2019 01:20

Chemistry, 26.06.2019 01:20

Mathematics, 26.06.2019 01:20

Mathematics, 26.06.2019 01:20


History, 26.06.2019 01:20


English, 26.06.2019 01:20

Mathematics, 26.06.2019 01:20

Physics, 26.06.2019 01:20

Biology, 26.06.2019 01:20

Mathematics, 26.06.2019 01:20

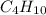
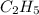 Empirical mass =
Empirical mass =