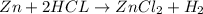When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipitate, zinc chloride (zncl2) was produced. she found that the mass of the zinc chloride was 93 g. since the law of conservation of matter stated that matter can't be created or destroyed, she was puzzled about the difference in mass. what is the best explanation for the missing 2 g?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipita...
Questions

Health, 25.03.2021 19:20




Spanish, 25.03.2021 19:20



English, 25.03.2021 19:20



Arts, 25.03.2021 19:20

Chemistry, 25.03.2021 19:20

Social Studies, 25.03.2021 19:20


Mathematics, 25.03.2021 19:20




Social Studies, 25.03.2021 19:20