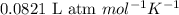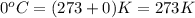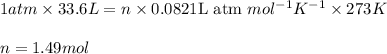Chemistry, 26.07.2019 06:50 mazolethrin3461
How many moles of oxygen (o2) are present in 33.6 l of the gas at 1 atm and 0°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1


Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
How many moles of oxygen (o2) are present in 33.6 l of the gas at 1 atm and 0°c?...
Questions


Mathematics, 04.03.2021 17:20



Physics, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20

Health, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20


Engineering, 04.03.2021 17:20

Social Studies, 04.03.2021 17:20

English, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20

Mathematics, 04.03.2021 17:20


Mathematics, 04.03.2021 17:20


Mathematics, 04.03.2021 17:20